Syndromic Surveillance Report
[NCIPC] Performance Monitoring of CDC’s Comprehensive Suicide Prevention Program
Att F 0920-1368 CSP Syn Surv Rpt
Syndromic Surveillance Report
OMB: 0920-1368
Attachment F – Syndromic Surveillance Report
Form Approved
OMB NO: 0920-1368
Exp. Date: 09/30/2025
Public reporting burden of this collection of information is estimated at 2 hours per response, including the time for reviewing instructions, searching existing data sources, gathering, and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/Information Collection Review Office, 1600 Clifton Road, NE, MS H21-8, Atlanta, GA 30333; Attn: PRA (0920-1368).
|
Comprehensive Suicide Prevention Project Syndromic Surveillance Report |
Instructions: The Comprehensive Suicide Prevention
(CSP) Project requires recipients to provide information about
syndromic surveillance activities and use of syndromic
surveillance data in the jurisdiction. Information for the entire
state is preferred, but the minimum requirement is to provide
information for the recipient's jurisdiction (e.g., state,
counties) identified for the project. Recipients must complete the
3 tabs in this report each quarter of the funding period (NSSP
Counts, Trends in DAPs, and Data to Action & Data Quality).
This report should be used to confirm syndromic surveillance
trends, assess the data quality of syndromic surveillance data in
the jurisdiction, and use of syndromic surveillance findings with
engaged partners. Submission deadlines are listed in the next tab.
Please take some time to share the information on all three tabs.
Thank you! |
|
Specific Syndromic Report Instructions: For ED visit counts, please confirm the count obtained by CDC by using data from the NSSP ESSENCE Biosense Platform. Suicide Syndromic Surveillance Definitions included in each submission are the SDC Suicide Related (v1), CDC Suicidal Ideation (v1), and CDC Suicide Attempt (v1 and v2). All of these definitions are incorporated into NSSP ESSENCE for use and applied to specific fields. CSP programs are able to use homegrown systems, modify these syndrome definitions, and use different parameters when conducting syndromic surveillance, however, CSP programs must use these same syndrome definitions and query parameters in NSSP ESSENCE to confirm the counts in this report. More details on query parameters will be shared when each report is shared with CSP programs for completion each quarter. Feel free to share any feedback your program has on the use of available syndrome definitions and specified query parameters. |
|
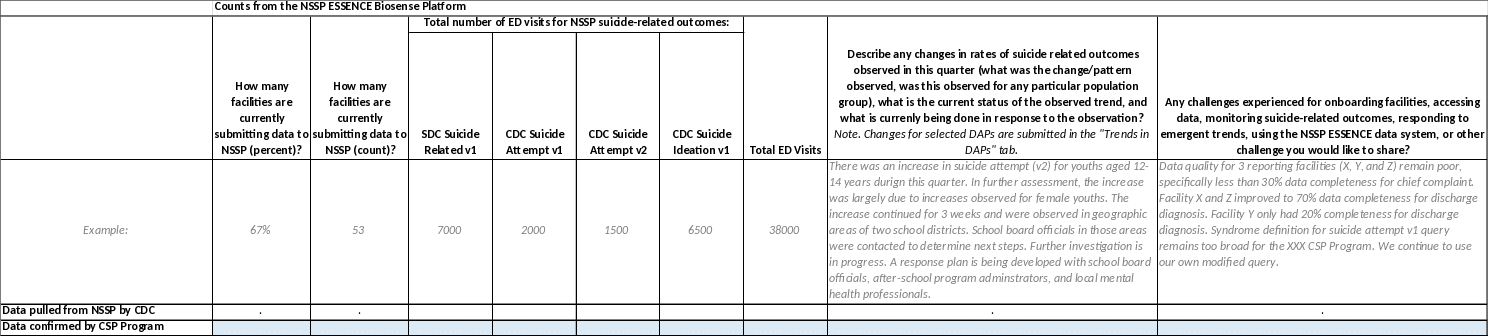
Instructions for the NSSP Counts Tab: Please fill in
the blue-colored cells in the "Data confirmed by CSP Program"
row to indicate how many facilities in your state/jurisdiction is
submitting data to NSSP (percent and count), what the total number
of ED visits were for the indicated quarter, and how many ED
visits there were in this quarter that met critiera for the NSSP
syndrome definitions for SDC Suicide Related, CDC Suicide Attempt
v1, CDC Suicide Attempt v2, and CDC Suicidal Ideation. In column
I, provide details on any changes in rates of suicide related
outcomes observed in the specified quarter, what change/pattern
was observed, was this observation for any particular population
group, and any other information helpful to understand the
observed syndromic surveillance trends. In column J, please
describe any challenges experienced for onboarding facilities,
accessing data, monitoring suicide-related outcomes via the
syndrome definitions, responding to an increases/upticks/emerging
trends, or other information you would like to share about data
validation, data quality, suicide syndrome definitions, using the
NSSP ESSENCE system, monitoring/responding to observed trends.
|
|
Instructions for the Trends in DAPs Tab: Please fill in the
blue-colored cells to indicate in rows 4 through 7, as possible.
Depending on each CSP program's selected DAPs, you may or may not
have the ability to use the NSSP ESSENCE system to monitor trends
for these populations. If your program is able to conduct
syndromic surveillance focusing on your selected DAPs, please
complete this tab. Each row is for one of your selected DAPs. In
column F, please describe any challenges experienced when
accessing data for this DAP, monitoring suicide-related otcomes,
responding to emergent trends, or other. |
|
Instructions for the Data to Action & Data Quality Tab: Please fill in the blue-colored cells to share what actions have been taken from the state team as well as what partners were engaged in response to syndromic surveillance findings. Please also list any available dissemination products, links, or trainings developed by your CSP program by sharing the url web links or emailing the documents to your CSP science officer and the csp@cdc.gov mailbox when you submit this report. |
Submission Guidelines
*CDC will pull data the week before the targeted date listed below. When this report is shared with CSP programs via e-mail, they should submit the completed report back to CDC by the due date specified in the e-mail (tentatively planned due dates are listed below). Note that the dates to include in each report is also listed for each submission. |
||
|
|
|
Targeted Date Sydnromic Report Template Will Be Sent to CSP Programs |
Tentatively Planned Date of Quarterly Report Submission by CSP Programs to CDC |
Dates Included in the Report |
April 1, 2023 |
April 15, 2023 |
(Oct 1, 2022 - Dec 31, 2022) |
July 1, 2023 |
July 15, 2023 |
(Jan 1, 2023 - Mar 31, 2023) |
October 1, 2023 |
October 15, 2023 |
(Apr 1, 2023 - Jun 30, 2023) |
January 1, 2024 |
January 15, 2024 |
(Jul 1, 2023 - Sept 30, 2023) |
April 1, 2024 |
April 15, 2024 |
(Oct 1, 2023 - Dec 31, 2023) |
July 1, 2024 |
July 15, 2024 |
(Jan 1, 2024 - Mar 31, 2024) |
October 1, 2024 |
October 15, 2024 |
(Apr 1, 2024 - Jun 30, 2024) |
January 1, 2025 |
January 15, 2025 |
(Jul 1, 2024 - Sept 30, 2024) |
April 1, 2025 |
April 15, 2025 |
(Oct 1, 2024 - Dec 31, 2024) |
July 1, 2025 |
July 15, 2025 |
(Jan 1, 2025 - Mar 31, 2025) |
October 1, 2025 |
October 15, 2025 |
(Apr 1, 2025 - Jun 30, 2025) |
January 1, 2026 |
January 15, 2026 |
(Jul 1, 2025 - Sept 30, 2025) |
April 1, 2026 |
April 15, 2026 |
(Oct 1, 2025 - Dec 31, 2025) |
July 1, 2026 |
July 15, 2026 |
(Jan 1, 2026 - Mar 31, 2026) |
October 1, 2026 |
October 15, 2026 |
(Apr 1, 2026 - Jun 30, 2026) |
January 1, 2027 |
January 15, 2027 |
(Jul 1, 2026 - Sept 30, 2026) |
April 1, 2027 |
April 15, 2027 |
(Oct 1, 2026 - Dec 31, 2026) |
July 1, 2027 |
July 15, 2027 |
(Jan 1, 2027 - Mar 31, 2027) |
October 1, 2027 |
October 15, 2027 |
(Apr 1, 2027 - Jun 30, 2027) & (Jul 1, 2027 - Sept 30, 2027) |
General ED Visit Counts

Trends in DAPS


Data to Action & Data Quality
Question |
Example |
Answer from CSP Program |
Please describe any actions taken from the state team or engaged partners in response to syndromic surveillance findings? |
The XXX CSP program launched a new website for men residing in rural areas. Partners and key stakeholders were informed of concerning increases in suicide attempts of rural adult male residents. Partners are currently discussing outreach campaigns to address these upticks. A committee was formed to plan an investigation for a suspected suicide cluster among in counties XX, XX, and XX. |
|
Please describe any syndromic surveillance dissemination products (e.g., dashboards, reports, factsheets, data briefs, publications, newsletters, etc; please provide url links to any available products)? |
New website for youths, this product was in response to a recent uptick identified by the NSSP alert system (available to the public): https://www.xxx.com |
|
|
Quarterly data brief (available only to partners, stakeholders, reporting facilities): http://www.xxx.com |
|
|
Syndromic surveillance training to improve data quality (available only to reporting facilities): http://www.xxx.com |
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Moore, Shamia (CDC/NCIPC/DIP) |
| File Modified | 0000-00-00 |
| File Created | 2025-09-23 |
© 2026 OMB.report | Privacy Policy